Heavy hydrocarbons
Heavy hydrocarbons

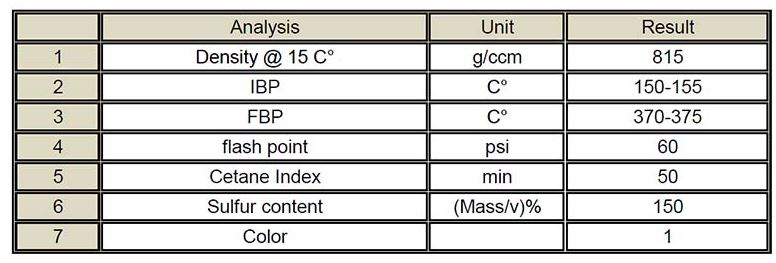
One of the refinery’s inter-distillation fuels, which has a distillation range of about 150 degrees Celsius to 385 degrees Celsius and performs well in domestic and industrial burners in diesel-powered engines, is called heavy hydrocarbon.
Hydrocarbons contain various organic compounds mainly made of H and C. In these molecules the carbon-hydrogen bond is a covalent bond (no ion bond).
Sulfur (S), nitrogen (N) and oxygen (O) atoms are usually substituted for carbon in hydrocarbons. The higher the amount of sulfur in the hydrocarbon compounds, the more it will change physical behavior and cause corrosion. The compounds get heavier and the oil refines harder. The less the substitutes in the hydrocarbons, the lighter the crude oil is.
Heavy hydrocarbons are derived from a combination of materials that have a flash point above 40 degrees Celsius, such as 400 family solvents, with refined industrial oils. The raw materials are first shipped from the refinery (non-flammable or raffinate solvent, HAB isozyricol, etc.) by tanker and discharged in 300,000 liters tanks then the oil required by the production lines to the tanks with the formulas provided in The laboratory is injected and fully pumped through the garbage pumps and then quality controlled by the loading lines in the tankers..